Marcus Chown in his book ‘The Magic Furnace’ elegantly describes how the different atoms build from Hydrogen in the furnaces of the universe. Through various thermal and nuclear reactions all of the elements from Hydrogen to Uranium are formed and the abundance of individual elements is inversely proportional to atomic mass. This distribution appears to be universal and is approximately reflected in the composition of our planet. Thus lighter elements are abundant in the Earths crust and atmosphere with lessening proportions of heavier elements. There are anomalies however: For example Helium,is certainly not abundant, but Helium is not chemically reactive and, being lighter than air, it is lost into space.
Beyond the atomic mass of lead all of the elements have radio-active isotopes and it is the kinetic energy released in radio-active decay of elements to stable lead which keeps the core of the earth in molten state. There are actually two starting elements in the radio-active decay to lead: Uranium and Thorium and the decay chains of both include elements which are gaseous. At this point it might be worthwhile to digress and to reflect upon how and why unstable nuclei decay:
With the exception of Hydrogen all nuclei contain positively charged protons with a similar, but usually greater, number of uncharged neutrons. The number of protons in a nucleus –atomic number (Z) – determines which element it forms and the total number of protons and neutrons defines the atomic mass (A). The number of neutrons for a given number of protons is subject to variation resulting in elements of different atomic mass – isotopes of the particular element. The Hydrogen nucleus alone can be without a neutron but its isotope nuclei, Deuterium and Tritium, have one or two neutrons respectively. Electrons, equal in number to the protons, orbit the nucleus in defined orbits and those in the outermost orbit determine how the element behaves chemically.
Neutrons act to dilute the powerful electrostatic repulsion forces between protons and this, combined with a stronger mass deficit force, prevents the nucleus from disintegrating. However for nuclides beyond Lead these forces are insufficient to keep nuclei intact and elements are radioactive. Thankfully unstable nuclei disintegrate randomly by small discrete steps (decay) such that new elements are formed sequentially. If the nuclei of newly formed elements are unstable they exist for periods ranging from fractions of a second to thousands of years. The quantity present of a decaying nuclide reduces exponentially with time so it is customary to describe its temporal decay in terms of half-life.
The primary decay step for heavy nuclides is the energetic emission of particles of mass 4. These alpha particles, which comprise two protons and two neutrons, are the nuclei of Helium atoms. Within a sequence of mass 4 decay steps to lead elemental ‘back steps’ occur –whereby a neutron transmutes to a proton by the emission of an energetic electron (beta particle). For these steps A is unchanged but Z increases by one.
The radioactive emission of particles often leaves the nucleus and its surrounding electron system in an excited state and particle emission are often accompanied by the emission of packets of electro-magnetic radiation (photons) as the new nuclide relaxes to the ground state. Photons emerging from the nucleus are gamma rays and those due to relaxation of the surrounding electron system are x-rays. Apart from origin, they are essentially the same but the former are usually of higher energy and are therefore more penetrating.
The heavy radio-active elements and their daughter elements are mainly concentrated in the earth’s core but small quantities occur to surface levels in all kinds of strata with the highest concentrations in areas of igneous rock such as granite. These together with cosmic rays from space produce an ever present low level background of ionising radiation under which life has evolved. We are tolerant of this low level of radiation unless in some way we manage to accumulate radio- active material within our bodies. Even so our bodies naturally contain some radio-active material through the Potassium in our blood; natural Potassium containing a small proportion of one of its radio-active isotopes, Potassium 40.
Alpha particles, although very energetic, are relatively massive and hence not very penetrating. In fact they are unable to penetrate the dead layer of our skin and alpha emitting nuclides are not considered to be harmful externally. Within our bodies it is a different matter particularly if alpha emitting isotopes enter the blood stream via our lungs. Like anything else radio-active material can enter our bodies through cuts and abrasions, ingestion or respiration but only the latter presents a significant threat and of which the only real possibility is when naturally occurring heavy nuclides are in a gaseous phase of their decay sequences – namely Radon and Thoron.
Radon and Thoron get into household air through diffusion from the ground and to a lesser extent from the materials from which houses are built. With good ventilation it is unlikely to be of any real significance- except possibly in areas with granite substrata- but, with the need to conserve energy, we tend to seal ourselves in more than hitherto and methods for preventing the ingress of Radon are incorporated into modern building regulations. In the light of this, the current craze for granite worktops seems a bit incongruous!
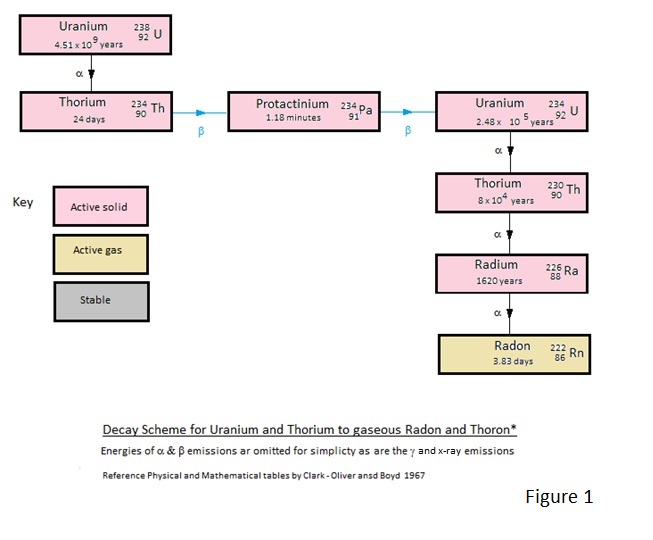
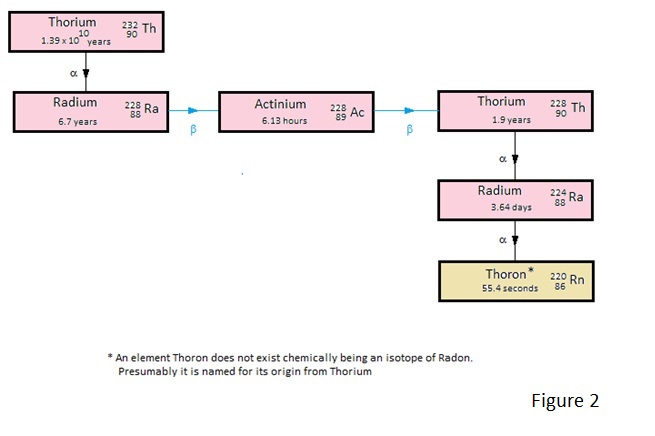
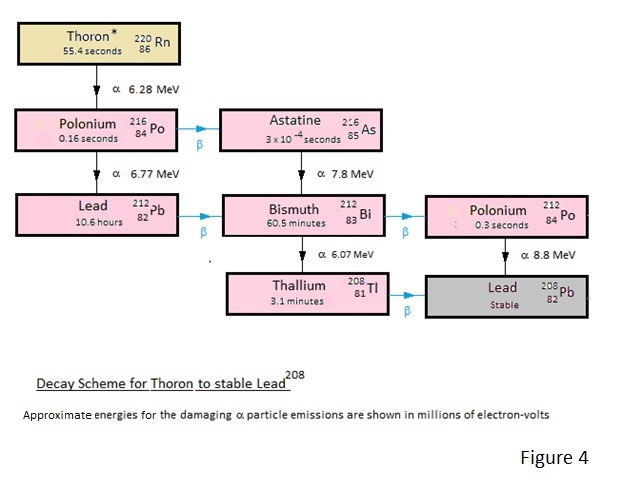
How the natural Uranium and Thorium decay to the Radon and Thoron and more importantly how these decay to lead.
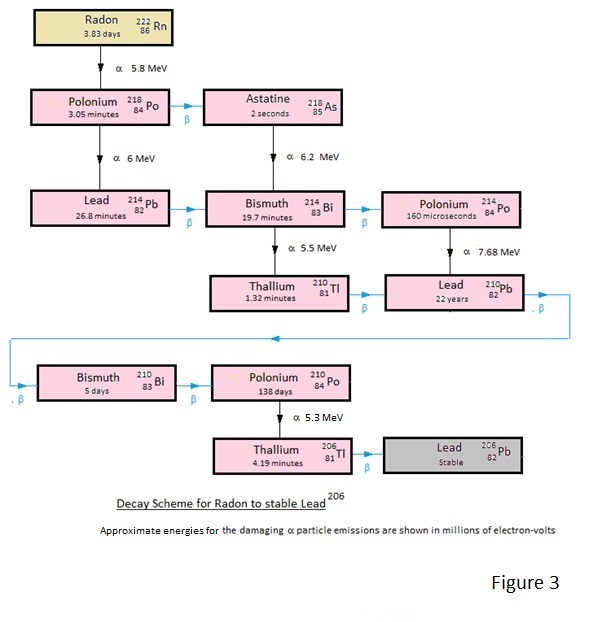
For the interested reader the decay schemes are depicted in Figures 1 to 4.
Of these Figures 1 and 2 show the decay schemes from Uranium and Thorium to gaseous Radon and Thoron and figures 3 and 4 show the more important decay schemes from these gases to stable lead isotopes.
 |
 |
| Because of its longer half-life Radon is more biologically significant than Thoron. |
Thank you for reading. |
| All information is free to use. |